Institutional Review Board

Responsibility
The primary responsibility of the Roseman University IRB is to safeguard the rights and welfare of human subjects who participate in research conducted under the auspices of the university and to ensure that subjects are aware of their rights and protections available to them.
The University’s human research protection program is based on three basic ethical principles described in the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The three principles are respect for persons, beneficence and justice. The application of the following principles can be found in the Belmont Report.
The IRB is charged with the review of all research projects that involve humans. The IRB has the authority to approve, require modifications (to secure approval), or disapprove all research activities that fall within its jurisdiction as specified by both the federal regulations and institutional policy.
Composition
The IRB is comprised of individuals from across the Roseman campuses with clinical and research expertise. Non-affiliated and non-scientific members represent the concerns of the community outside of Roseman.

IRB Training
Members of the IRB and investigators must complete a Human Subjects Research course from the Collaborative Institutional Training Initiative (CITI). To register and complete the CITI training click on CITI button below.
Click here, to view instructions on how to create an account and select the required IRB course(s),
Click here, to watch a video demonstrating how to create an account and select the required IRB course(s),
Submissions
Roseman University uses a web-based submission system, IRBManager. It offers electronic submissions and reviews, automatic notifications; web-based protocol sharing and collaboration, integrated training and credential management. To create or access an IRBManager account, click on IRBManager picture below or go to https://roseman.my.irbmanager.com
Click here, to view the Quick Start Sheet to get started with IRBManager.
Click here, to view a video that helps with getting started with IRBManager.
IRB Bulletins
IRB FAQ
For additional information please visit our IRB FAQ section by clicking the questions below.
Any research activity involving human subjects or data of human subjects conducted on the premises of Roseman University, or conducted by Roseman University investigators, needs to be in compliance with the policies and guidelines set forth in the Roseman IRB Policy Manual 2024.
The federal regulations define research as “a systematic investigation designed to develop or contribute to generalizable knowledge.” Quality assurance, quality improvement projects or program evaluations may not fall under the Institutional Review Board jurisdiction. Any feedback requests or clarification requests must be submitted in writing to irb@roseman.edu.
The federal regulations define a human subject as “a living individual about whom an investigator (whether professional or student) conducting research 1) Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens, or 2) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
The IRB recommends that the Principal Investigator (PI), who is a faculty member, draft and submit the protocol as the individual who will assume primary responsibility of the proposed research investigation. Roseman students may be involved as part of the research team. All members of the research team are under the supervision of the faculty member, as the PI.
Roseman Faculty and staff are considered “Affiliates” in IRBManager and can be the PI on a protocol. Students, Residents, and Non-Affiliates (individuals who do not have an appointment at Roseman) cannot be the PI on a protocol.
Click here, for an outline of the Initial Submission form to allow for students to help collaborate.
Yes. The standardized forms for IRB submission and templates for supporting documents are accessible in IRBManager. The forms in IRBManager are “smart” forms and do not need to be downloaded, they can be completed and submitted within the IRBManager system.
Roseman Investigators – Must complete the Human Subjects Research course by Collaborative Institutional Training Initiative (CITI). To register and complete the CITI training click on the image below.
For instructions on creating an account and selecting the correct courses, click here.
Be sure to use your Roseman email address to allow your CITI account to be linked to your IRBManager account.
External Investigators – investigators not affiliated with Roseman University must either demonstrate that they meet their institution’s human subject research training policies, by submitting the institutional policy and the training certificate, or take Roseman’s required CITI training.
Roseman investigators must complete either Human Subjects Research – Biomedical and/or Human Subjects Research – Social & Behavioral CITI training.
Investigators involved in clinical trials, regardless of funding source, must take the appropriate Good Clinical Practice CITI training module. Investigators of protocols funded by the National Institutes of Health (NIH) or National Science Foundation (NSF) must also take the appropriate Responsible Conduct of Research CITI training module.
Click here, to view instructions on how to create an account and select the required IRB course(s).
Click here, to watch a video demonstrating how to create an account and select the required IRB course(s)
Roseman Investigators – If your Roseman email address is associated with your CITI account, the CITI training should be automatically linked to your account. If your training is not, please contact the IRB Office.
External investigators – upload the institutional policy and the training credentials on the ‘Results and Additional Info’ page of the application.
All Roseman University Faculty Investigators, must create an IRBManager account to 1) Demonstrate completion of CITI Human Subject Research training, 2) Access, and monitor the research project review progress. External investigators and Roseman University students can be added as ‘contacts’ on protocols to demonstrate completion of the required CITI training.
If you had an account and protocols on IRBNet – email the IRB Office to have your login information sent to you. Registering via this method will allow for your protocols in IRBNet to be transitioned to your new account on IRBManager.
If you did not have an IRBNet account – click on the image below and select ‘Click here to register.’ which is located in the bottom left hand corner of the ‘Login’ box.
Be sure to use your Roseman University email address when signing up for an account in IRBManager. IRBManager only accepts @roseman.edu email addresses.
Click here, to view the Quick Start Sheet to get started with IRBManager.
Click here, to view a video that helps with getting started with IRBManager.
Log into IRBManager and click on “Start xForm” to start a new Initial Submission Application. To access forms for studies that are already approved, click on the study on the dashboard and then click “Start xForm.” Accessing xForms within the study will allow you to complete the Adverse Event Reporting Form, Closure Form, Continuing Review/Annual Check-In Form, and Modification Request Form.
The submission is reviewed for completeness and internal consistency. Based on that administrative review, the investigator may receive a ‘Changes Requested’ email asking for corrections, clarifications, or additional documents. The next step is review based on the level of IRB review (see below).
Click on the link in the email to be directed to your protocol in IRBManager. Address all requested revisions outlined in the email. IRBManager automatically tracks what changes you made, so there is no longer a need to complete a Revisions letter.
The review and approval time will vary significantly based on the level of review and other factors such as turn-around time by the PI in response to the administrative review, Board review, and complexity of the project among others. It is important that the PI plans accordingly.
For Exempt Review, expect the Administrative review to take up to 1 week. We will send either approval or a ‘Changes Requested’ email. The PI will then determine how quickly the process can go depending on how soon she/he addresses the changes requested. Reviewing the submitted revisions can take up to an additional week. Depending on the quality of the revisions, the PI can receive an additional ‘Changes Requested’ email, if more changes are needed, or it will be forwarded to the IRB Chair for final review and determination.
For Expedited Review, expect the Administrative review to take up to 1 week. If the protocol requires changes a ‘Changes Requested’ email will be sent. The PI will then determine how quickly the process can go depending on how soon she/he addresses the changes requested. When the administrative review is completed, the protocol will be sent to at least two IRB Members for review. We give IRB Reviewers 2 weeks to complete their review, as IRB Members are volunteers who donate their time. After the IRB Reviewers complete their review, the IRB Office may send out one final Changes Requested email based on the most recent review and recommendations. Once the requested revisions have been made, the protocol will then be sent to the IRB Chair for a final review which can take up to 1 week. If the chair does not require changes, a letter of approval will be sent.
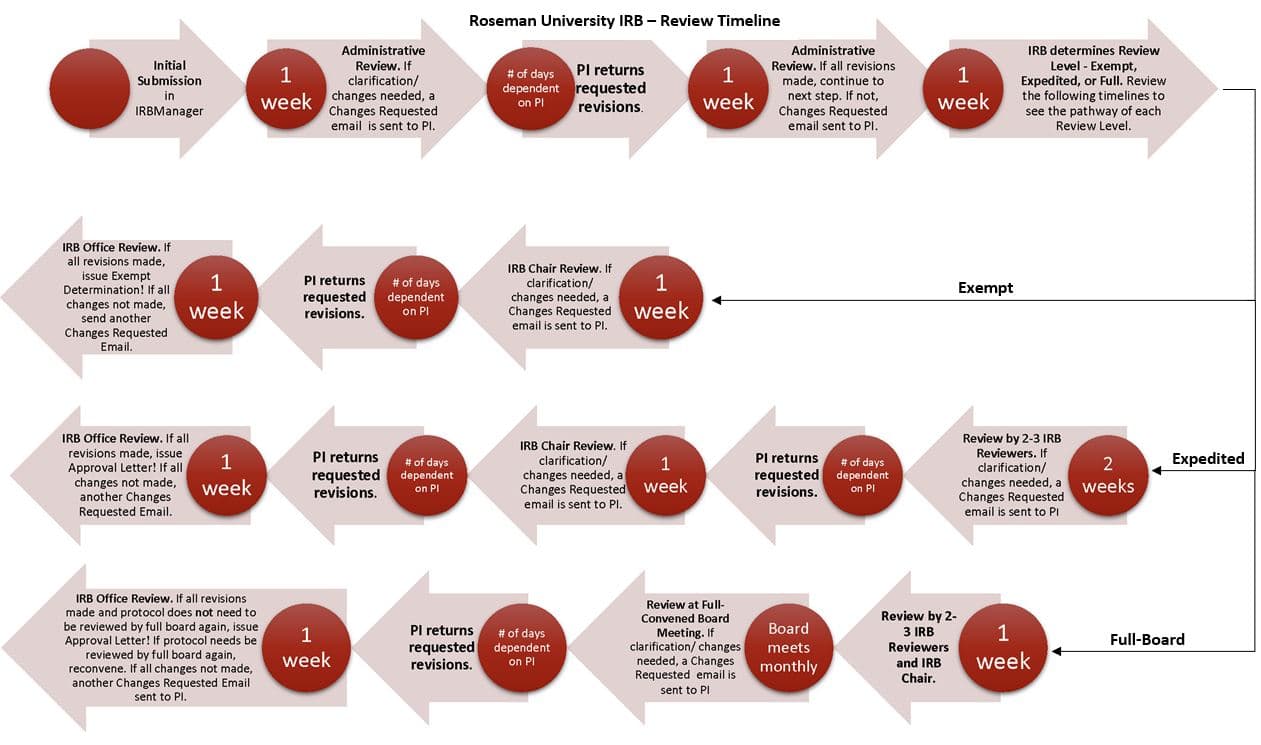

Full IRB meetings are scheduled monthly.
Under no circumstances may a research investigation be started before the IRB decision on approval or exemption is received. To check protocol review status, login to your IRBManager account.
Yes, if any incident of injury, adverse effects, or unanticipated problems experienced by subjects in research takes place, the incident must be reported to the IRB via the Adverse Event Reporting Form.
Also, if any modifications need to be made to the protocol, the PI must submit a Modifications Form on IRBManager. Login to IRBManager, select the study on the Dashboard and click “Start xForm” to be directed to the Modification Form.
Regardless of risk level, all protocols must undergo an Annual Check-In. Protocols that were determined more than minimal risk must undergo a Continuing Review. The PI will receive notifications regarding the required Annual Check-In 60, 30, and 1 day prior to expiration.
Email irb@roseman.edu.